
"AN INVESTMENT IN KNOWLEDGE ALWAYS PAYS THE BEST INTEREST" BENJAMIN FRANKLIN

"AN INVESTMENT IN KNOWLEDGE ALWAYS PAYS THE BEST INTEREST" BENJAMIN FRANKLIN

Short Communication - (2022) Volume 59, Issue 2
Received: Jun 13, 2022, Manuscript No. BSSJAR-22-66535; Editor assigned: Jun 16, 2022, Pre QC No. BSSJAR-22-66535; Reviewed: Jun 30, 2022, QC No. BSSJAR-22-66535; Revised: Jul 07, 2022, Manuscript No. BSSJAR-22-66535; Published: Jul 14, 2022, DOI: 10.36962/GBSSJAR/59.2.008
Metal corrosion causes enormous harm to many industries, primarily to oil and gas production and processing enterprises. This is explained by the aggressive properties of corrosive environments while oil producing, which are conditioned with the presence of a large amount of mineralized water, hydrogen sulfide and sulfate-reducing bacteria, as well as carbon dioxide in them. One of the most economical and effective methods of protecting metals is associated with the use of corrosion inhibitors (Syrquin 1988).
Inhibitors are used at all stages of processing, transportation of oil, gas and petrochemical products in these industries. The protection of metals from corrosion by inhibitors is based on the property of certain specific chemical compounds or their mixtures to reduce the rate of the corrosion process or completely suppress when they are introduced in insignificant concentrations into a corrosive environment. In this regard, the problems of studying corrosion processes and measuring the corrosion rate by taking polarization curves become urgent. An increase in the reliability of equipment can be achieved by carrying out anti-corrosion treatment of pipeline systems (Kuznets 1973).
Electrochemical methods for studying the process of corrosion of steel samples, which consist of the removal of polarization curves on a potentiostat are known. Curves graphically reflecting the dependence of the anode and cathode currents on the potential are called polarization curves (Olczyk 2018). The stationary state of the system corresponds to the value of the potential at which the condition of equality of the anode and cathode potentials is satisfied.
The assessment of the fundamental possibility of electrochemical corrosion is made by the magnitude of the corrosion element EMF, which is equal to the difference in the equilibrium potentials of the conjugated electrochemical reactions. It is convenient to represent the kinetic characteristics of corrosion processes graphically in the form of a set of polarization curves of cathode and anode processes. At the same time, the potentials of anode and cathode processes are plotted along the abscissa axis, the voltage of the corresponding anode and cathode currents is plotted along the ordinate axis, or a semi -logarithmic dependence is plotted in the coordinates "potential-lg current density".
Judgment about the real rate of the corrosion process can be obtained from the kinetic dependences of the anode and cathode reactions. Cathode and anode polarization curves are recorded directly on the sample, the corrosion of which is being studied. The intersection point of the anode and cathode polarization curves determines the corrosion rate on the abscissa axis, and the stationary potential on the ordinate axis. Since near the stationary potential the polarization data cease to fit into a semi- logarithmic dependence, the corrosion rate is usually found from the intersection point of the extrapolated rectilinear curves (Bah 2011). The value of the stationary (corrosion) potential always lies between the values of the equilibrium potentials of coupled reactions. The general corrosion rate is expressed by the current per unit area of the entire metal surface (current density) without dividing it into cathode and anode sections. The corrosion rate depends, interalia, on the mechanism of anodic dissolution of the metal and the cathode reduction of the oxidant, and thus can be calculated depending on the relative position of the polarization curves (Mallick 2017).
The graphical method for calculating the corrosion rate allows, in contrast to the analytical method, to calculate the corrosion rate for complex cases corresponding to the real conditions of the corrosion process.
The aim of this work is to measure the corrosion rate by taking polarization curves (Michaely 1962 and Cortuk 2011).
Experimental technique
To study inhibitor action mechanism of the hydrogen sulfide corrosion of steel MARZA-1, a potentiostatic method for measuring polarization curves was used.
Polarization measurements were carried out on a stationary electrode reinforced with epoxy resin cured by polyethylene polyamine, having a working area of 0.36 cm2 in a potentiostatic mode (potentiostat P-5827 m) in a three-electrode cell with separate cathode and anode spaces. Electrode from comparise in is auxiliary silver chloride-smooth platinum. The potentials are recalculated according to the Normal Hydrogen Scale (NHS) atmosphere-air.
The inhibition coefficients of the anode  electrode reactions were calculated at a shift from Ecor by 20 mV according to the formula (1).
electrode reactions were calculated at a shift from Ecor by 20 mV according to the formula (1).

Where,  - are the densities of the anode or cathode current in the absence and presence of an inhibitor, respectively. Polarization was carried out from the cathode to the anode region with a holding time of 30 s at each potential.
- are the densities of the anode or cathode current in the absence and presence of an inhibitor, respectively. Polarization was carried out from the cathode to the anode region with a holding time of 30 s at each potential.
A simulated formation water MI was used as a background solution to perform corrosion tests. Before testing, the samples were washed with distilled water, and then placed in a 500 ml beaker so that the selected area was completely immersed in the MI formation water imitator (Kavakiotis 2017, Goodall 2009 and Kozlenkova 2014).
Experimental part
Analysis of potentiostatic curves showed, the MARZA-1 inhibitor predominantly slows down the anode process that in the presence of low concentrations of H2S (50 and 100 mg/l) (Figure 1).
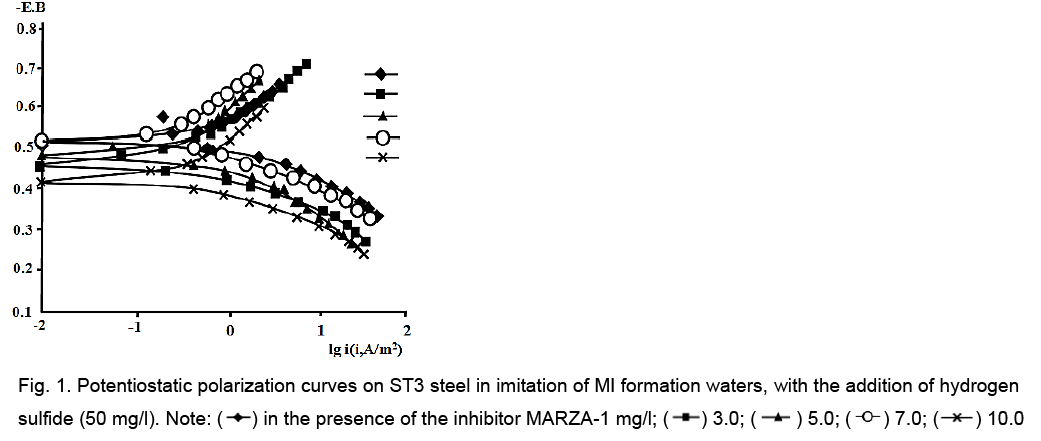
Figure 1: Potentiostatic polarization curves on ST3 steel in imitation of MI formation waters, with the addition of hydrogen sulfide (50 mg/l). Note:
Increase in hydrogen sulfide concentration up to 400 mg/l leads to the fact that, at a concentration of 5.0, 7.0 and 10.0 mg/l, MARZA-1 inhibits both electrode reactions, and at a concentration of 3 mg/l, only anodic, in the absence of influence at the cathode (Figure 2).
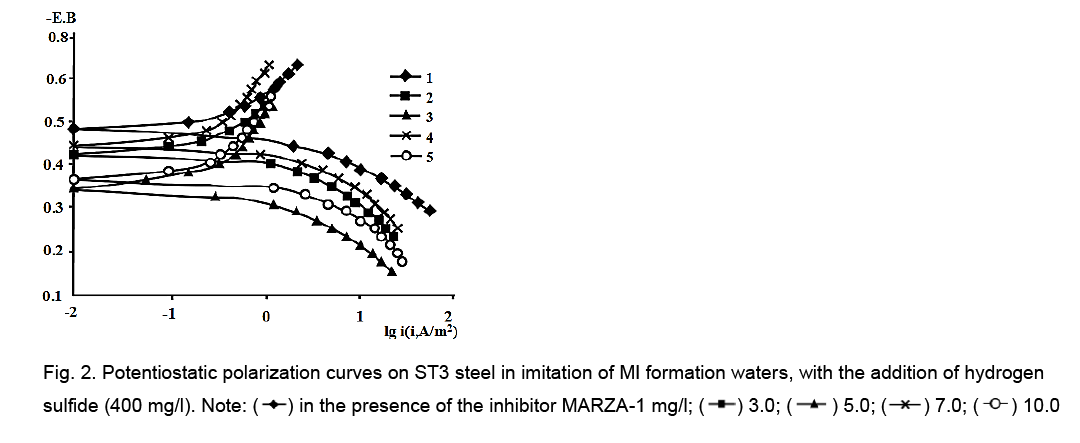
Figure 2: Potentiostatic polarization curves on ST3 steel in imitation of MI formation waters, with the addition of hydrogen sulfide (400 mg/l). Note: in the presence of the inhibitor MARZA-1 mg/l;
in the presence of the inhibitor MARZA-1 mg/l;
For the first time the effect of the inhibitor "MARZA-1" on the corrosion rate of steel in the MI water formation imitates was studied. Corrosion rate is measured by taking polarization curves. It was found that the investigated inhibitor "MARZA-1" effectively slows down both cathode and anode reactions in the presence of hydrogen sulfide in the model formation water MI. The inhibiting efficiency of a reagent called "MARZA-1" in relation to hydrogen sulfide corrosion of steel in model formation water MI was studied by electrochemical methods (by taking polarization curves). It was found that the investigated inhibitor "MARZA-1" effectively slows down both cathode and anode reactions at the electrode in the presence of hydrogen sulfide in the model formation water MI.
[Crossref] [Google Scholar] [Pubmed]